News Archive
RESEARCH HIGHLIGHT – Effects of pharmacological modulators of α-synuclein and tau aggregation and internalization
Parkinson's disease (PD) and Alzheimer's disease (AD) are common neurodegenerative disorders of the elderly and, therefore, affect a growing number of patients worldwide. Both diseases share, as a common hallmark, the accumulation of characteristic protein aggregates, known as Lewy bodies (LB) in PD, and neurofibrillary tangles in AD. LBs are primarily composed of misfolded α-synuclein (α-syn), and neurofibrillary tangles are primarily composed of tau protein. Importantly, upon pathological evaluation, most AD and PD/Lewy body dementia cases exhibit mixed pathology, with the co-occurrence of both LB and neurofibrillary tangles, among other protein inclusions. Recent studies suggest that both α-syn and tau pathology can spread and propagate through neuronal connections.
The OMG-GOE team developed a simple laboratory model system to investigate the mechanisms underlying aggregation and propagation of these proteins, with the goal of informing the development of novel therapeutic strategies. In their study published in Nature Scientific Reports in July 2020, they assessed the effects of molecules on the aggregation and internalisation of tau and α-syn and identified some that can decrease α-syn and/or tau aggregation. Establishing the effects of small molecules with different chemical properties on the aggregation and spreading of α-syn and tau will be important for the development of future therapeutic interventions.
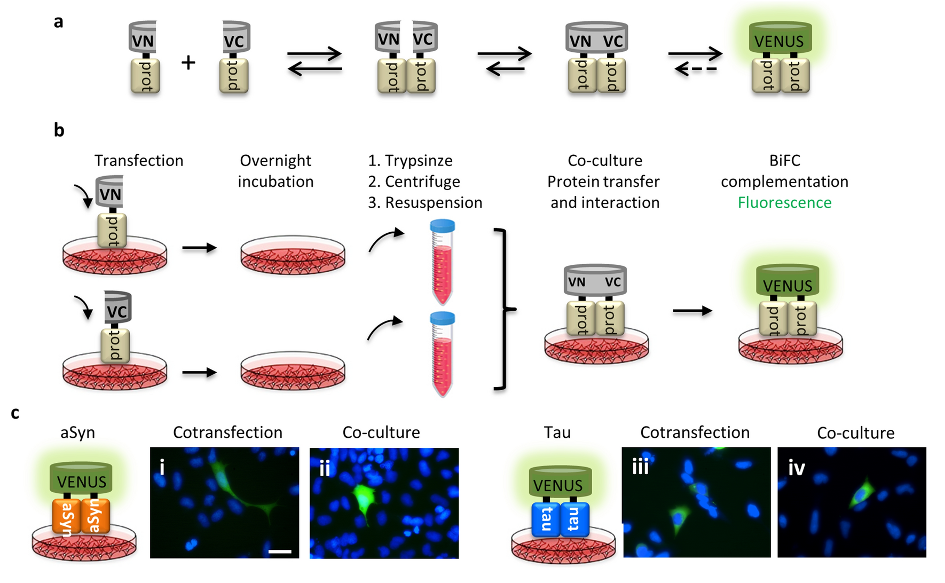
The initial steps of protein aggregation can be monitored by the bimolecular fluorescence complementation assay (BiFC), based on the reconstitution of the Venus fluorescent protein. This assay enables us to monitor the release and uptake of proteins by cells, and to test the effect of different molecules, such as the ones described in our study.
This project receives funding from the Innovative Medicines Initiative 2 Joint Undertaking (www.imi.europa.eu) under grant agreement No 116060. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA.
This work is supported by the Swiss State Secretariat for Education‚ Research and Innovation (SERI) under contract number 17.00038.
The opinions expressed and arguments employed herein do not necessarily reflect the official views of these funding bodies.



