News Archive
RESEARCH HIGHLIGHT - Cryo-EM structures of tau filaments from Alzheimer's disease
Alzheimer’s disease is defined by abundant plaques and tangles in cerebral cortex. Tangles are made of abnormal amyloid filaments of post-translationally modified microtubule-associated protein tau. Paired helical filaments (PHFs) were first identified in Alzheimer’s disease brain in 1963. The less abundant straight filaments (SFs) were described later. Between 1985 and 1992, PHFs and SFs were shown to be made of all six human brain tau isoforms. It was suggested that the microtubule-binding repeats of tau form the filament core, with the remainder forming the fuzzy coat. Until the recent resolution revolution in electron cryo-microscopy (cryo-EM), it was not possible to obtain high-resolution structures of non-amplified tau filaments from brain.
In 2017, IMPRiND partner UCAM reported the first high-resolution structures of amyloid filaments from human brain. These findings, Cryo-EM structures of tau filaments from Alzheimer's disease, published in Nature, show that the Alzheimer tau fold can form in the absence of extracellular amyloid deposits. Each tauopathy appears to have its own fold, but the same fold can be found in multiple tauopathies.
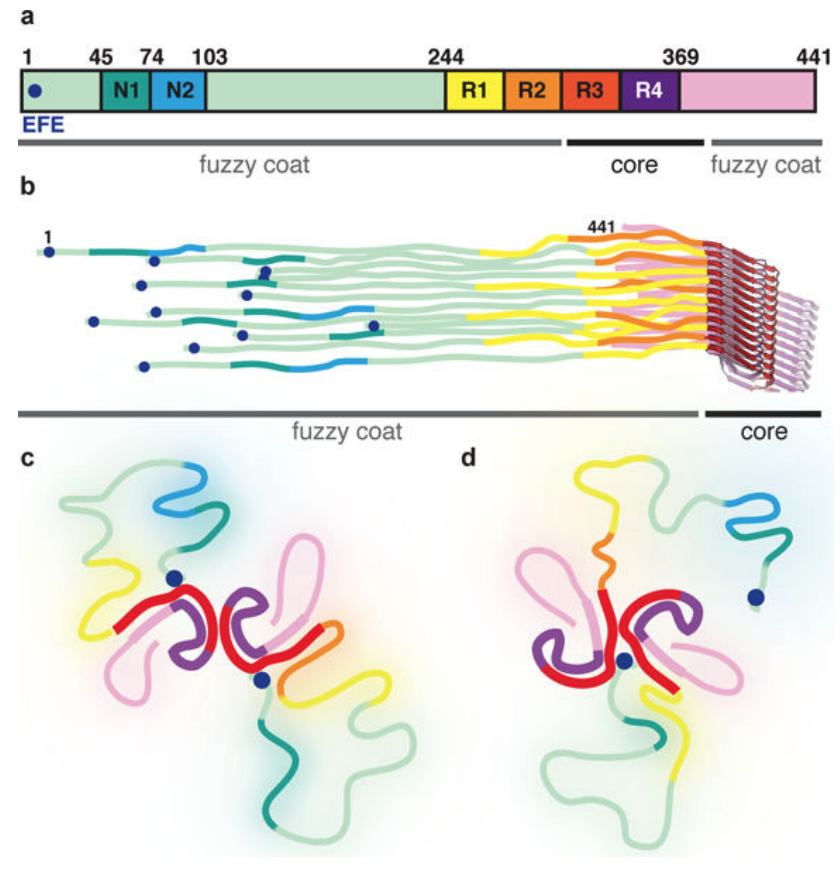
Schematic representation of full-length Tau filaments
This project receives funding from the Innovative Medicines Initiative 2 Joint Undertaking (www.imi.europa.eu) under grant agreement No 116060. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA.
This work is supported by the Swiss State Secretariat for Education‚ Research and Innovation (SERI) under contract number 17.00038.
The opinions expressed and arguments employed herein do not necessarily reflect the official views of these funding bodies.



